About Event
Welcome to the 5th Optimizing Hybrid Clinical Trials Summit, the only case-study driven forum dedicated to understanding ROI, costs, and potential cost efficiencies of running DCTs, in addition to building financial cases for internal stakeholders to run remote trials. This meeting will provide actionable data on how other companies have succeeded or been set back to inform the best strategies for executing DCTs.
Why Attend?

Explore case studies to gain approval from internal executives to initiate a hybrid clinical trial Bayer

Examine ways to streamline and negotiate site coordinator costs to identify cost-saving incentives when running a hybrid clinical trial Veterans Health Foundation

Create cost efficiencies through strategic sourcing and procurement to elevate ROI and strengthen vendor relationships Jazz Pharma

Overcoming data centralization issues faced by clinical trial sites when adopting digital endpoints AbbVie

Use pilot-scale strategy for assessing implementation of novel technology to improve patient experience and engagement in hybrid clinical trials Astellas
Hear From Our Past Participants:
“I enjoy the smaller conferences that enable discussions. Also, a good line up of speakers who are experts in their field.”

“Informative, innovative, and organized! Great conference. I learned so much!”

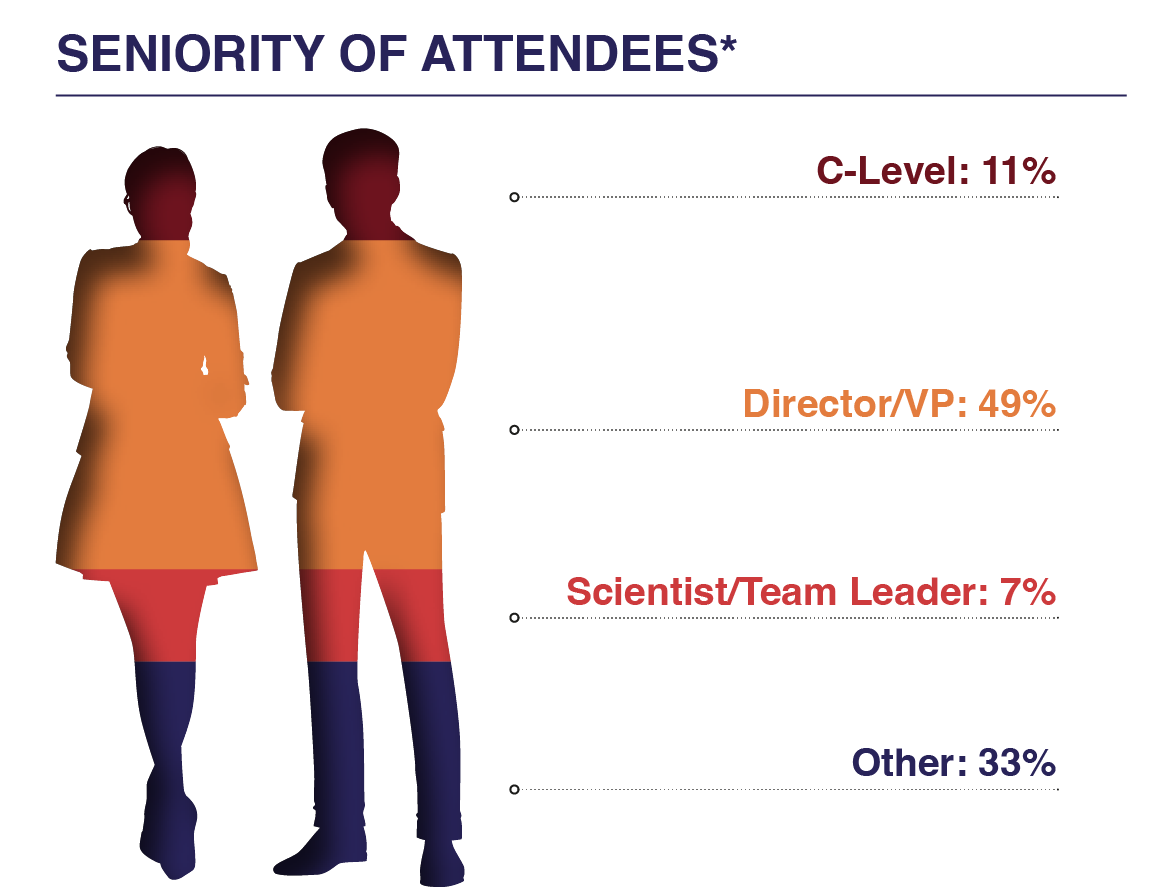
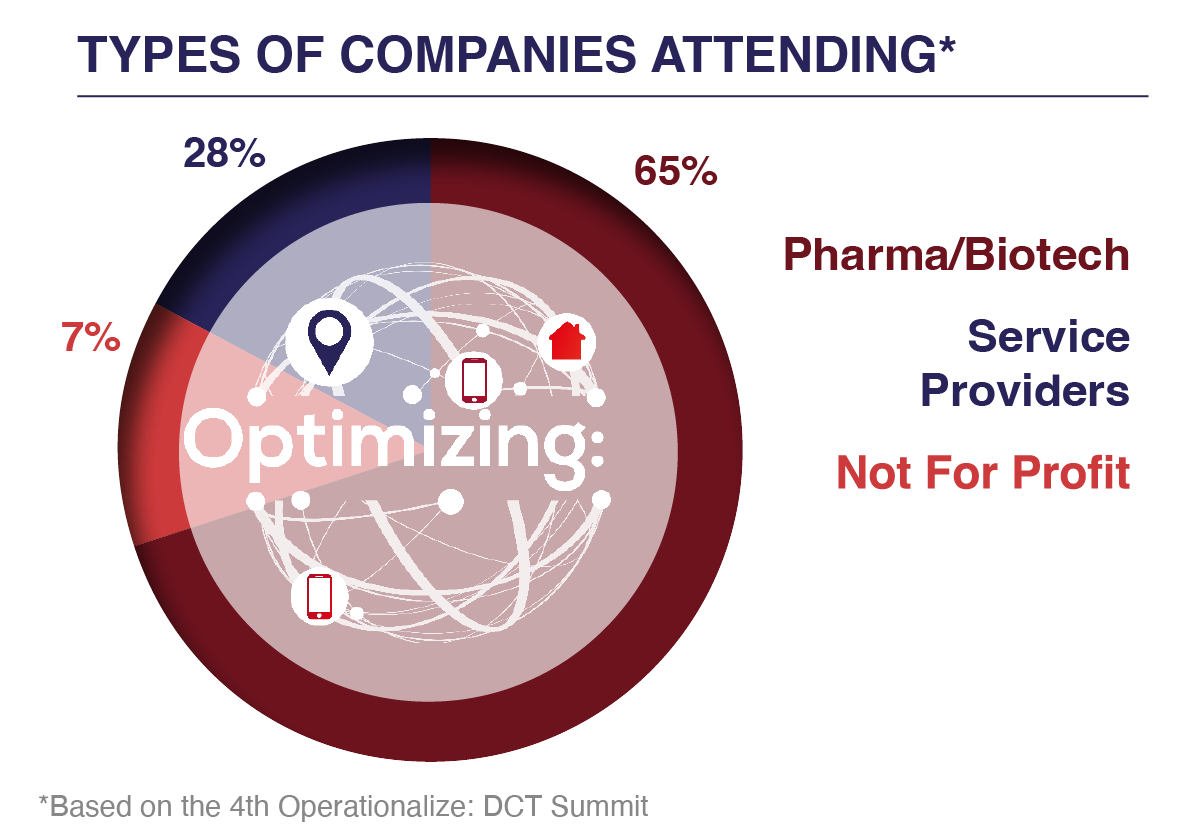
Who Attends?